Abstract
Objectives
Methods
Results
Conclusions
Objectives
To evaluate the spectrum of diagnostic findings in hysterosalpingography (HSG) examinations performed at our institution between 2006–2010 and their prognostic significance for treatment decisions and fertility outcomes.
Patients were filtered from our PACS. Pathological HSG studies were re-evaluated. Indications for referral, technical success and diagnostic findings were analysed. Pathological findings were correlated with further diagnostic workups, treatments and fertility outcomes.
Of 411 HSG examinations, 226 (55 %) were normal, 94 (23 %) showed minor abnormalities and 5 (1.2 %) were not diagnostic. Eighty-six (21 %) examinations were pathological. Twenty-nine patients underwent subsequent laparoscopy, during which proximal tubal occlusion diagnosed at HSG was ruled out in 9/23 cases. Follow-up information was unavailable for 20 patients. Nineteen of 66 patients with follow-ups after pathological HSG had at least one subsequent successful pregnancy. Forty-one patients had no further treatment and no pregnancies.
Conclusions
The detection rate for pathologies at HSG was low (21 %). There was a high false-positive rate (39 %) for proximal tubal occlusion, most likely because of spasms, demonstrating the importance of delayed imaging after injection of antiperistaltic agents. HSG remains a valuable diagnostic tool. Our results, however, indicate that this technique should be more selectively indicated.
Main Messages
• Good acceptance of HSG by the patients. No complications with antibiotic prophylaxis.
• Low detection rate (21 % pathological exams) for pathologies in our study.
• High false-positive rate for proximal tubal occlusion.
• This demonstrates the importance of waiting longer after injection of buscopan.
• High pregnancy rate in pathological cases: Indication too broad or even a therapeutic effect of HSG?
Introduction
Approximately 15 % of couples are affected by infertility, which is defined as the inability to conceive after 12 months of regular unprotected sexual intercourse [1]. Common causes of infertility include male factor (45 %), ovulation disorders (37 %) and tubal damage (18 %) [2]. A combination of several factors is found in approximately 20 % of all couples. The etiology of tubal damage can be intrinsic (ascending salpingitis, including salpingitis isthmica nodosa) or extrinsic (peritonitis, endometriosis and pelvic surgery). The most common causes of pelvic inflammatory disease (PID) are Chlamydia trachomatis, Neisseria gonorrhoeae and multibacterial infections [3]. Studies have demonstrated that the severity of tubal damage found in infertile women is directly related to their serum chlamydia antibody IgG titer (CAT) [4].
Uterine cavity abnormalities can be a contributing cause of subfertility in 10 % of women. Abnormal uterine findings are reported in as many as 50 % of women with recurrent implantation failure [5]. These findings include endometrial polyps or fibroids, which are observed as filling defects or uterine wall irregularities using hysterosalpingography (HSG). HSG can also demonstrate intrauterine adhesions and congenital abnormalities [1].
Imaging plays a key role in the diagnostic evaluation of female infertility [6]. Transvaginal ultrasound (TVUS) is a standard, first-choice procedure. Abnormal findings can be further evaluated with saline or contrast hysterosalpingo sonography [1]. Hysterosalpingo contrast sonography (HyCoSy) has been found to be highly sensitive, specific and accurate in identifying uterine abnormalities, e.g., polyps. However, it is of limited value for the assessment of tubal abnormalities. Magnetic resonance imaging (MRI) can be used to evaluate congenital Müllerian duct anomalies and to diagnose adenomyosis, leiomyoma and endometriosis; however, its role in tubal assessment is presently limited [7, 8].
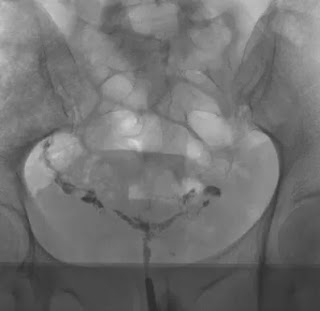
The primary role of HSG is to evaluate the morphology and the patency of the fallopian tubes. The fallopian tubes should appear as thin, smooth lines that widen in the ampullary portion. Tubal abnormalities observed with HSG can be congenital, or due to spasm, occlusion or infection. Tubal occlusion manifests as an abrupt cutoff of contrast material with non-opacification of the distal fallopian tube, and can be unilateral or bilateral. Peritubal adhesions prevent contrast material from spilling into the abdominal cavity and distributing freely [8, 9].
HSG can also be helpful in evaluating uterine cavity abnormalities. It is considered to have a high sensitivity (60–98 %) but low specificity (15–80 %) in detecting uterine abnormalities, and hysteroscopy remains the method of choice for the final assessment. The differential diagnosis of intrauterine filling defects by HSG includes polyps, endometrial hyperplasia, submucosal fibroids, intrauterine adhesions and septa. These findings necessitate further investigation with hysteroscopy to confirm and possibly treat the pathology [1]. In the literature, the results of HSG studies have been shown to be important for selecting patients for
diagnostic laparoscopy with chromopertubation [9, 10].
In 2008, guidelines published by the European Society of Human Reproduction and Embryology (ESHRE) recommended that a semen analysis and an ovulation assessment should be performed before a tubal patency test. Women with a high probability of pathological conditions should be offered a first line laparoscopy. This approach has the advantage that any tubal or pelvic pathology can be investigated and treated at the same time. The ovaries can be assessed by TVUS according to the ESHRE guidelines [11, 12]. Following these guidelines, we advise women to undergo a first line laparoscopy combined with hysteroscopy if they have a history of previous pelvic surgery, PID, elevated CAT, or severe dysmenorrhea or dyspareunia.
The purposes of this study were to evaluate all pathologies that were diagnosed by HSG in the workup of female infertility at our institution between 2006 and 2010, to describe the spectrum of diagnostic findings, and to correlate the diagnostic findings with clinical findings and outcomes.
Patients and methods
Patients were identified by searching the radiology information system (RIS) for all HSG examinations from September 2006 (introduction of our Picture Archiving and Communication System; PACS) to April 2010. A total of 411 HSG examinations were identified and included in the study. The ages of the patients ranged between 22 and 42 years, and the mean age was 32.6 years.
For most patients, diagnostics and treatment were performed in our department. Some patients were sent to our department only for tubal assessment by their gynaecologists or family doctors. In selected cases, follow-up information concerning pregnancy or pathological findings was therefore not available.
Our department of gynecology specialises in the diagnosis of infertility and its treatment, including in vitro fertilisation (IVF). The Department of Gynecology as well as the Institute of Radiology are certified by DIN EN ISO 9001:2000. Approximately 450 intrauterine insemination (IUI) cycles and 450 fresh/frozen IVF/intracytoplasmatic sperm injection (ICSI) cycles were performed.
Results
No immediate, i.e., allergy to the iodinated contrast agent or propfol, or delayed, i.e., pelvic infections, complications were observed during or following any of the 411 examinations. Of the 411 HSG examinations, 226 (55 %) were normal. In 15 of the examinations that were assessed as normal, administration of Buscopan® was required to differentiate spastic tubal occlusion from true tubal occlusion. There were minor abnormalities observed in 94 examinations (23 %). Five examinations (1.2 %) were not diagnostic (early termination of the exam due to venous filling of the uterine plexus).
In 86 (21 %) of the examinations, at least one pathology was described that was probably or possibly relevant to female infertility (Table 1). The detailed analysis of our study was based on this subpopulation. There was a statistically significant age difference between patients with normal (mean 31.8 years) and pathological (mean 34.1 years) HSG examinations (P-value = 0.0001).
Of these 86 patients, 30 solely uterine pathologies were identified. The most common uterine pathologies were filling defects (n = 15, Figs. 2 and 3) and Müllerian duct anomalies (n = 11), including arcuate uterus, hypoplastic uterus, uterus septus (Fig. 4) and uterus bicornis bicollis (Fig. 5a and b). A few patients (n = 4) had minor abnormalities of the uterine cavity that were described as likely originating from mucosal irregularities (Table 1).
Additionally, 29 laparoscopies (LSC) and 19 hysteroscopies (HSC) were performed on 34 different patients. Three additional pathologies of the uterine cavity (2 small polyps and a single myoma) were identified that were not visible in the HSG. Nine of the 23 one- or two-sided proximal tubal occlusions diagnosed by HSG were normal at laparoscopy, resulting in a false-positive value of 39 %. On the contrary, HSG showed a high negative predictive value (100 %): No additional tube pathologies were identified at LSC if the tube morphology was normal at HSG. In the nine patients mentioned above, LSC (n = 5) and HSC (n = 4) were combined with an intervention (tuboneostomy, myoma resection or one-sided salpingectomy).
A very common procedure following a pathological HSG was insemination because of male subfertility. This procedure was performed as long as one of the fallopian tubes was described as normal and the uterine cavity had no major pathology.
In total, there were 27 pregnancies in 25 of the 86 patients (including 20 patients lost to follow-up, see below) with a pathological condition at HSG. These pregnancies resulted in the birth of 19 healthy children and 8 miscarriages.
Seventeen of the 25 patients only bore a healthy child. These 17 patients had the following HSG results: one-sided tubal occlusion (n = 6), adhesions (n = 4, one of these with uterus arcuatus), filling defects (n = 5), tubal irregularities, possibly postinfectious (n = 1) and sactosalpinx (n = 1).
Two of the 25 women had miscarriages followed by successful pregnancies. Both of these patients showed filling defects, and one patient had additional tube irregularities, which were possibly postinfectious.
Six of the 25 patients had pregnancies that resulted in spontanteous abortions. In these six patients, the HSG showed one-sided tubary occlusion (n = 2), two-sided tubal occlusion (n = 2), adhesions (n = 1) and an arcuate uterus (n = 1).
Results of further diagnostic workup
Eleven of the 86 patients had no further diagnostic workups. Three of the 86 patients had diagnostic workups following HSG without surgical procedures or further pathologies identified.
Six of the 86 patients that solely had an miscarriage as the outcome showed the following conditions:
- Two-sided tubal occlusion, normal LSC and HSC. Pregnancy after insemination (n = 1).
- One-sided tubal occlusion confirmed at LSC (n = 2), 1/2 with endometriosis at HSC.
- Two-sided tubal occlusion, fundus myoma at HSC not visible at HSG (n = 1).
- Two-sided tubal occlusion, abortion after ovum donation (n = 1). This patient had no LSC because of her relatively high age.
- Uterus arcuatus (ovarian insufficiency) (n = 1).
One of the 86 patients had a miscarriage that was followed by a successful pregnancy. In this patient, the HSG showed a cavity abnormality due to adenomyosis/synechiae. The HSC diagnosis showed multiple scars. An additional myoma that was not visible at HSG was resected (Fig. 3).
Four of the 86 patients had resection of a myoma (n = 3) or a polyp (n = 1) prior to becoming pregnant. Three of these HSG examinations identified filling defects, and one was not visible. One of these four patients had a spontaneous abortion prior to a normal birth. The other three patients gave birth to healthy children.
Forty-one of the 86 patients had no further treatment and no pregnancies. Two of these patients had no wish for further children; the examination was performed to assess the need for anticonception.
For 20 of the 86 (23 %) patients, follow-up information was unavailable. Approximately half of these patients were referred from outside institutions for HSG examinations only, and the remainder were drop-outs from the fertility program.
Discussion
In our fertility center, HSG is still the most common first-line diagnostic test to evaluate the uterine cavity and tubal patency. HSG is relatively easy to perform and can be completed as an outpatient procedure. Using a good anesthetic protocol with propofol sedation, the procedure is well tolerated by patients. Whereas the administration of Propofol obviously increases patient comfort, the procedure is performed without any sedation in many other institutions, and the advantages should be discussed in light of the additional costs.
As recommended by the ESHRE guidelines [11], we excluded other infertility causes, such as male or ovarian factors, before performing a tubal patency test. According to the ESHRE guidelines, a first-line laparoscopy/hysteroscopy should be performed when tubal, uterine or pelvic pathologies are suspected or detected by TVUS.
In recent years, CAT has become an important screening test that can be used to decide upon further examinations [10]. Hysterosalpingo contrast sonography (HyCoSy) has become an important tool for the diagnosis of uterine or tubal pathologies [12]. According to the literature, this technique has a higher sensitivity than HSG (0.80 versus 0.53) [13], and the specificity (0.84 versus 0.87) is similar for both exams [14].
In our opinion, HyCoSy is more demanding for the examiner than HSG, it is better for assessing the uterine cavity than the fallopian tubes , and it has a longer learning curve. The advantages of hysterosalpingo contrast sonography are that it costs less and causes less pain; therefore, no anesthesia is necessary. Additionally, there is no exposure to radiation.
Whereas HyCoSy and HSG only have diagnostic value, laparoscopy and hysteroscopy also have therapeutic options, including adhesiolysis, excision of endometriotic lesions or resection of intrauterine polyps.
Considering that HSG is far more expensive and invasive (i.e., anesthesia, exposure to radiation) than HyCoSy, our aim is to avoid unnecessary HSG exams. Since 2009, we have routinely used CAT screening to select patients for laparoscopy/hysteroscopy as the first-line exam, and we have set an age limit of 38 years, above which we perform HyCoSy instead of HSG.
During the years of our retrospective study from 2006 to 2010, we chose the diagnostic procedure according to the criteria shown in Table 2. One of the aims of our retrospective study was to improve our decision-making and to avoid unnecessary exams. Of the 411 analysed HSGs, 86 (21 %) demonstrated at least one pathology described as potentially relevant for fertility. This is quite a low detection rate, meaning that we performed four of five HSG procedures in vain. This clearly indicates that the indication for HSG at our institution in recent years was too broad. Furthermore, there was a high false-positive rate for proximal tubal occlusion (39 %) and a high pregnancy rate in patients with pathological HSG, i.e., 25/86 of the total patients and 25/66 of the patients with follow-up.
We acknowledge some limitations to our study. First, the retrospective study design might appear to be a limitation of this study; however, all of the images from the examinations had been archived in our PACS, and all pathological cases were re-evaluated. No follow-up information was available for 23 % of the patients with a pathological condition at HSG. It is not known how many of these patients became pregnant. Dropout rates have been reported in the literature to be as high as 50 % before the beginning of therapy. It is likely that patients interrupt their fertility care because of emotional distress and poor prognoses [15]. Furthermore, some follow-ups were not available in our study for patients sent to our center only for tubal assessments by their gynaecologists or family doctors.
Another limitation is that laparoscopic and/or hysteroscopic results were available in only 29 of 411 HSG patients. This means that there was no information about possibly false-negative exams in the patients with normal HSG exams. From the 86 HSG exams with suspected pathologies, 29 patients had laparoscopy/hysteroscopy, and 20 patients were lost to follow-up. Therefore, it is not known how many of these patients became pregnant.
In conclusion, HSG is a safe, low cost and, with the application of propofol sedation, a well-tolerated procedure for tubal assessment, which should be performed at the end of the infertility investigation protocol. The relatively low percentage (21 %) of pathological exams in our population underlines the need for good patient preselection. The high false-positive rate for proximal tubal occlusion (39 %), probably due to tubal spasm, demonstrates the importance of antiperistaltic agents and delayed imaging. Furthermore, we observed a high pregnancy rate (in patients with pathological HSG). Most cases of pregnancy were spontaneous without tubal or uterine surgery. This could mean that the indication for HSG was too broad or that there could even be a therapeutic effect of the HSG procedure, i.e., improved patency of the fallopian tube because of the flushing during the examination [16]. As a result of this data analysis and literature review, the workflow in our own center will be adapted. We will establish hysterosalpingo-contrast sonography (HyCoSy) as a first-line exam for tubal and uterine factors, and improve the patient selection for primary laparoscopy/hysteroscopy using routine screening for CAT.
Mohak Infertility Center is one of the Best Infertility treatment centre in indore. Dr Shilpa Bhandari – IVF specialist in Indore uses high medical techniques for investigation and counsels the patients. Book an appointment Today Call now 7898047572 For more information, visit - https://www.mohakivf.com
Please go through our social media :
like our page to no more about ivf
Please do follow on Instagram
Instagram : https://www.instagram.com/mohak_ivf/
To More Post: Coverage of infertility treatment and fertility outcomes







Nice blog Post.
ReplyDeleteIvf centre Hyderabad is a leading international destination for infertility treatment including icsi, IVF - in vitro fertilization, egg donation, embryo freezing and other fertility treatments.
Ivf centre Hyderabad
Ivf with egg donation in Hyderabad
Surrogacy services in Hyderabad
Thanks for sharing the Blog.
ReplyDeleteHegde Fertility is the Best Fertility & IVF Center in Hyderabad. A Name you can trust for IVF, IUI, ICSI and Endometriosis Treatment.
Best fertility center in Hyderabad
Natural Ivf treatment in Hyderabad
IUI cost in Hyderabad